Heisenberg Uncertainty Principle
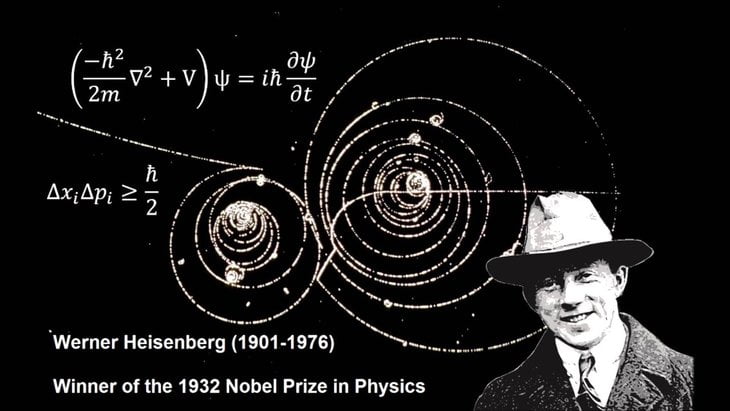
The Heisenberg Uncertainty Principle is one of the most fascinating and complex ideas in quantum mechanics. First introduced by German physicist Werner Heisenberg in 1927, this principle plays a crucial role in our understanding of the microscopic world, revealing the inherent limits of what we can know about the tiniest particles in the universe.
At its core, the uncertainty principle tells us that it's impossible to know both the exact position and momentum of a particle at the same time. Position refers to where a particle is located, while momentum combines its speed and mass. In everyday life, like when observing a moving car, we can easily determine both where it is and how fast it’s going. But in the quantum world, where particles like electrons exist, this level of certainty is unattainable.
The more accurately we try to measure a particle's position, the more uncertain its momentum becomes, and the same goes the other way around. Imagine trying to pinpoint the exact location of an electron. As we do this, we inevitably disturb its momentum, making it impossible to determine both properties precisely at the same time. It's as if the universe imposes a natural limit on our ability to gather complete information about these tiny particles.
This principle is more than just a theoretical curiosity—it fundamentally shapes how we understand the behavior of particles at the quantum level. For example, electrons in an atom don't orbit the nucleus in well-defined paths like planets around the sun. Instead, they exist in a cloud of probabilities, where we can only describe their likely locations rather than pinpointing an exact spot.
The Heisenberg Uncertainty Principle also challenges the classical idea that the universe is entirely predictable. In the world of classical physics, knowing an object’s position and momentum would allow us to predict its future behavior with great accuracy. However, the uncertainty principle introduces a level of unpredictability into the quantum world, showing that nature operates on probabilities rather than certainties.
This principle has significant implications not only for our understanding of the natural world but also for the technology we use every day. Modern electronic devices, such as smartphones and computers, rely on principles of quantum mechanics, including the uncertainty principle, to function. Moreover, emerging technologies like quantum computing leverage this uncertainty to perform calculations that are far beyond the capabilities of traditional computers.
In essence, the Heisenberg Uncertainty Principle reveals the limits of our knowledge about the smallest building blocks of the universe. It shows us that even in the most precise scientific inquiries, there are boundaries to what we can know. This principle has revolutionized our understanding of the microscopic world and continues to inspire both scientific exploration and philosophical reflection. It's a reminder that the universe is filled with mysteries, some of which may forever remain just beyond our grasp.
Comments
Post a Comment